UNDERSTANDING
RADIATION
RADIOACTIVITY &
IONIZING RADIATION

Introduction
All too often all that people know about radiation is its connection to the atom bomb and its massive destructive powers. The terms Radiation, Radioactivity, and Radioactive are often times used interchangeably, while not always technically correct. Further confounding this issue is another less-known term called Ionizing Radiation. In this article, we will peer into this invisible part of our universe to better understand what they are, their relationship to one another, and how they are different.
What is Radiation?
Let’s begin by first understanding what Radiation is. Radiation is the emission or transmission of energy in the form of waves or particles through space or through materials. The two energy forms, waves and particles, are important to understand so let’s explore each of these beginning with energy in the form of waves.
The word radiation arises from the phenomenon of waves radiating meaning traveling in all directions, from a source. One visible example of waves being radiated is when you drop an object onto a body of still water that produces waves emitted from the center and traveling outwards. Another great example is our sun, which radiates its energy outward in all directions.
When we think of the sun, we think of it radiating visible light, but in fact it also radiates infrared and ultraviolet light as well. The infrared, visible, and ultraviolet energies are all part of what is called the Electromagnetic Spectrum.
The Electromagnetic spectrum as represented on the accompanying chart is a range of energies measured by their frequency (in terms of Hertz or cycles per second), their wave lengths, and their photon energies.
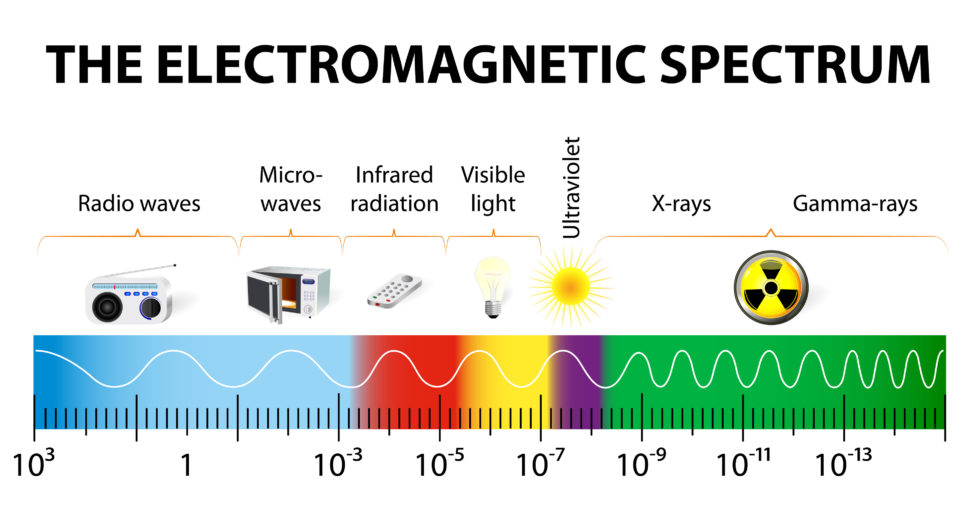
This chart shows the electromagnetic spectrum of waves and begins at the left side of the chart with low energy, long waves known to us as radio waves. Radio waves can be anywhere from one kilometer to 1,000 kilometers and more in length. As the energy increases, the wave frequency also increases with the wavelength becoming shorter and shorter until you arrive at the far-right side of the spectrum where the wave length is only a small fraction of the diameter of an atom and where the frequency and energy strength are both very high.
While we may not readily recognize these wavelengths, we do easily recognize the devices that operate at these different energy levels such as radios, microwave ovens, and infrared lamps. Going up in energy there is the very narrow visible energy band we humans are able to see with our eyes. After visible light, the energies progress on to ultraviolet, through x-rays, and ending with gamma-rays.
Man has been able to use the different frequencies to operate separate AM & FM radio broadcasting channels for our listening pleasure, navigation systems like LORAN, GPS, and beacons, communications for HAM radio, cell phones, walk i-talkies, secure military communications, emergency broadcasting, Morse code, Telecom and much much more.
What is Ionizing Radiation?
A primary concern is at what energy level across this entire spectrum do we begin to experience negative effects that cause damage to our bodies? More specifically, at what energy point do we see cell damage occurring in the tissues that make up our body? While this question continues to be vigorously debated today as in the case of cell phones, one clearer distinction is the energy level required that cause changes to the electrical charges within atoms.
Atoms as we know all have a delicate balance of protons, neutrons and electrons. Any radiation energy with sufficient power to knock off electrons results in an imbalanced charged atom known as an ion. Scientists have measured the radiation with enough power to knock off electrons at about 10 electron volts and higher and have classified them as ionizing radiation. Any radiations with insufficient power to knock off electrons are classified as non-ionizing radiation.
The ionizing region begins in the midst of the ultraviolet range of energies and then proceeds through the increasingly higher x-ray and gamma regions. That is why we see cautionary measures being exercised anytime we’re exposed to these type of ionizing radiations. Studies have shown that Ion Atoms with missing electrons results in an electrical imbalance that cause chemistry changes to occur to the molecules and also our DNA that make up our tissues and can lead to harmful outcomes.
Damage to cells vary by energy and the amount of exposure they receive. Ultra-violet energy is relatively low and does not have much penetration capability so damage is limited. X-rays and Gamma-rays on the other hand are both very powerful as they are more energetic and have deep penetrating power.
When exposed to low or intermittent levels of ionizing radiation our bodies are able to handle it and repair themselves. But when the exposures are too severe, our bodies cannot keep up with the rate of damage and we begin to experience radiation sickness, burns, and death in the most extreme cases.
What is Radioactivity?
This now leads us to the last term Radioactivity. Radioactivity is when alpha, beta, and neutron particles from inside an atom’s interior are spontaneously being emitted or ejected either because they are:
• Electrically unstable
• Being bombarded by man made accelerators
• Interacting with other radioactive materials as in the case of producing fission.
There exists a variety of radioactive elements that occur naturally here on earth like uranium, thorium, potassium, radium, and radon. Added to this are man made radioactive elements like plutonium, americium, californium, and einsteinium to name a few.
All of these radioactive elements emit particles as they strive to neutralize their energy in a process we call decay. Some elements will emit particles for fractions of a second before neutralizing and becoming non-radioactive; others can take up to millions of years.
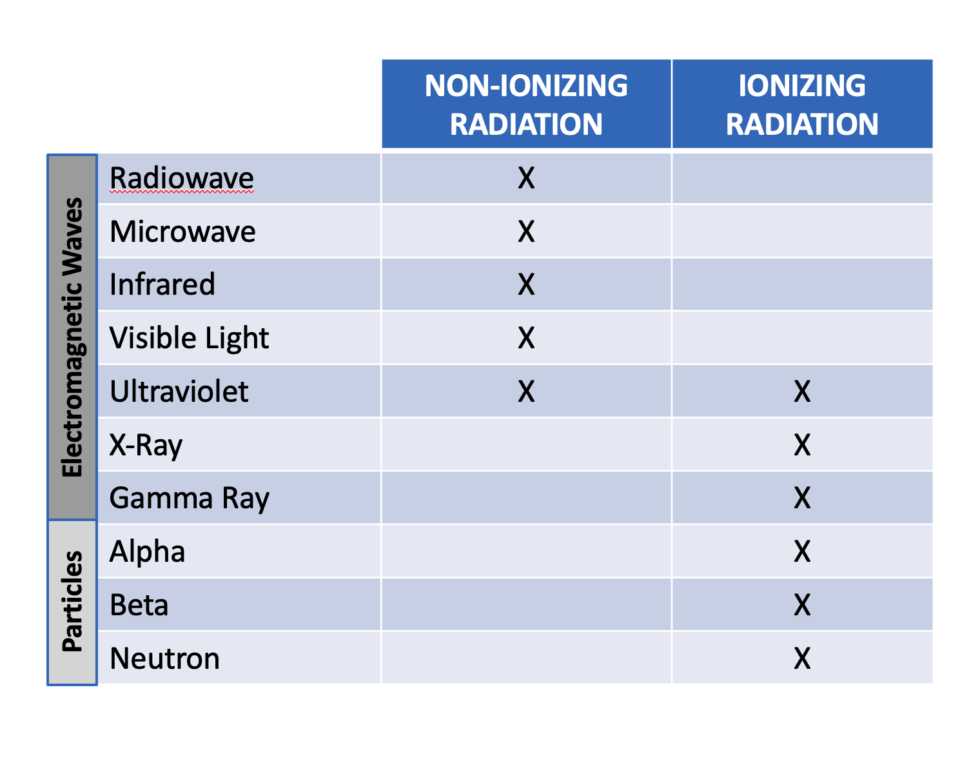
While the particles types and energies will differ they are classified as ionizing type of radiation along with their higher energy electromagnetic wave counterparts. The chart seen here gives us a good overview of radiation as a whole with distinction between particles and electromagnetic waves and between non-ionizing and ionizing classifications.
Conclusion
In conclusion radiation is energy being emitted or transmitted through space or materials like the tissue in our bodies. We are most concerned with ionizing type of radiation where the radiation energy has sufficient power to knock off electrons that can cause harmful effects to our bodies if we receive excessive exposures.
Electromagnetic waves in the form of x-rays and gamma-rays are ionizing radiation. So too are radioactive elements emitting alpha, beta, gamma and neutrons particles.
Knowing what these terms mean helps us to better understand the invisible realm all about us.

